Office of Research & Development |
 |

The revised Project Cover Sheet (PCS) wizard is available in the IRBNet sandbox the week of December 11. The updated wizard outline (VA network access only) is posted on the VAIRRS SharePoint portal under the ORPP&E Standard Form Library menu option. The VA Project Lead/Principal Investigator and Additional Project Personnel sections moved to the Study Team Tracking wizard. The IRB of Record Type will appear for all studies that select "Data and/or biospecimens from living human individuals." On the PCS PDF both the "Funding Source Code" and the "Funding Source Code - Other" response will now appear.
Study teams will not be required to update previous PCS submissions unless there has been a change to the study.

The auto-generated notifications can be customized for your site. Please contact IRBNet Support at govsupport@irbnet.org for more information.

Recusals can be noted on the Reviews and Minutes page. Under the Voting section, you can add or remove recused committee members, and the recused members will be recorded in the minutes.

During the May Change Control Board (CCB) meeting, the following requests were approved.
|
FORM/LETTER/WIZARD |
SUMMARY OF CHANGES |
|
3.81L Director Support Letter for Prisoner Research |
The numbering convention of the form was updated from 3.8L to 3.19L to correct a typo. |
|
3.5A RDC Non-Veteran Application |
Removed Section 3: Investigator Signature from the MS word and PDF versions of the 3.5A RDC Non-Veteran Application form. |
|
7.2A Combined ICF Form and HIPAA Authorization Template |
Language under the section, "WHAT ARE THE COSTS TO ME IF I TAKE PART IN THIS STUDY," was updated as follows: from "You will not be charged for any treatments or procedures..." to "You or your insurance will not be charged for any treatments or procedures..." |
|
7.3A Informed Consent Template |
The IRBNet Library Access Instructions document has been replaced with the new Standard Form Library Access Instructions User Guide. This updated guide is now available on the Standard Form Library within the VAIRRS SharePoint and IRBNet.

The VAIRRS Strategic Advisory Council (VSAC) recently met to review current personnel tracking processes. The VSAC determined personnel tracking should occur separately from project coversheet tracking. This will provide a more efficient means of updating study personnel and associated training requirements. The VAIRRS team is working on a draft personnel tracking form. We will provide further updates as development progresses.

The COI and RCO Modules are available for all facilities to use. If your facility is interested in adopting either module, please reach out to IRBNet Support at govsupport@irbnet.org.

Reminder: New Project Submission Forms were the latest forms to be revised and were released on October 25, 2023. All New Project Submission Forms must be used as of January 2, 2024. Prior to using these new forms, please read the Summary of Changes – CIRB New Project Forms for a detailed list of forms and process changes. For additional information regarding these changes, you may also refer to the VAIRRS webinar from October 24, 2023, titled “CIRB Forms and Process Updates.”

In June, VAIRRS University added the VAIRRS FSR Dashboard training video series. This series consists of five micro-training videos designed to help VA field staff navigate and access data for active projects.
Users can access the training videos within VAIRRS University by navigating to the Training Material category and selecting "Training Videos." In this category, there is a folder titled, "VAIRRS Field Staff Reporting (FSR) Dashboard Training Videos" (VA network access only). An accompanying VAIRRS Field Staff Reporting Navigation Guide (VA network access only) is also available on the VAIRRS FSR Dashboard (VA network access only).
To learn more about VAIRRS University, watch the recording of the VAIRRS University walk-through webinar, led by VAIRRS experts.
For questions about VAIRRS University, contact the VAIRRS Support Team at VAIRRS@va.gov.

VAIRRS is planning to host a COI summer training series to support investigators and study teams on the use of VAIRRS to document COI per VHA DIRECTIVE 1200.13. Look out for more information in the coming weeks.

The VAIRRS Mentor Program currently has four team members from various roles across VA providing mentorship, with the ability to support more mentees.
If you are interested in getting help from a fellow VAIRRS user or becoming a VAIRRS mentor, access the mentee/mentor applications via the VAIRRS Mentor Program SharePoint page (VA network access only). Applications for mentees are accepted on an ongoing basis throughout the year. Applications for mentors are accepted on an ongoing basis until all slots are filled.

The VAIRRS Support Team is expanding the "Expired Projects Email Notification" email to include Project Status and IRB Project Risk Level discrepancies. Expect the new "Data Integrity" email format to launch in mid-June.

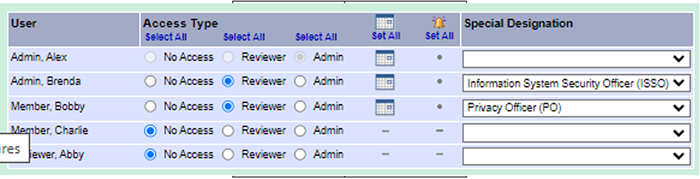
When assigning a package for ISSO (information system security officer) or PO (privacy officer) review in the research administration or R&DC (Research & Development Committee) workspaces be sure to select the appropriate reviewer designation from the drop-down list. The ISSO and PO reviewer designations are very helpful when tracking reviewer activity.

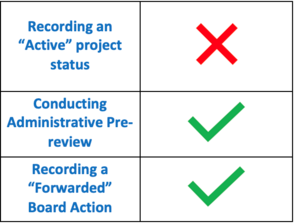 |
Did you miss this month’s VAIRRS webinar, “How to Properly Utilize the Research Admin Workspace in IRBNet”? If so, you are encouraged to review the webinar recording and available training resources. A summary of the activities discussed at the webinar are highlighted in the table to the left. |

The ORD Focused Inquiry Navigation Database (FINDPro) (VA network access only) tool helps users find information regarding ORD policy related to human, animal, and laboratory research protections. The latest FINDPro updates include:
Please note: The ePROS webpage is also undergoing changes and the URLs will soon be changing from ORPP&E to ePROS. In addition, some content will be reorganized to reflect the new structure of the enterprise transformation. When this happens, please resave your bookmarks. Sorry for the inconvenience.

Recent revisions to the combined Informed Consent Form (ICF)/HIPAA Authorization include the following that begin on page 8 and extend through page 10. This includes language clarifications from VHA Privacy.
As of April 10, 2024, study teams who previously completed an STT Wizard will be required to re-enter the first and last names of all "Additional Personnel" the next time they revise the STT Wizard.
Note, this is a one-time occurrence that will prompt users to revise their STT Wizard the first time they return to their existing STT Wizard following this update.
For any study team that completed the STT Wizard before the Wizard update on the evening of April 10, 2024. When you open the "Additional Project Personnel" page, all names of previously entered personnel on this page will be blank. However, the rest of each person's entry is still completed (contact information, duties, etc.). To know which person's name to fill in the two new fields, scroll down to the "VA email" field, which should identify the correct name. Another option would be to download the previously submitted STT Wizard from Designer and use it to re-enter the names of the additional personnel.

Principal Investigator's (PI) concerned with sharing their or their team’s outside compensation with the rest of the team or the R&D committee can now use an alternative reporting solution, provided below.
Short-term workaround for existing projects:
Rationale:
The R&DC is not required to review outside salaries for existing projects, therefore the submission of information can take place outside of VAIRRS. The workaround meets the
requirement for posting the approval memo to the project file, and the only individuals with access to the salary information are the PI who submits, the ACOS, and the Authorizing Official. The R&DC members with access to the project will also have access to the internally published memo. If this is a concern, the Committee Administrator can remove all committee member access or just provide access to the Chair.

The Office of Research Reviews (ORR) has exciting news for the VA research community. As indicated in the March VAIRRS update, ORR is developing a series of information security instructions to support the research community. To continue the series, ORR developed a one-page quick reference guide (QRG) that informs research teams about common ORR findings that result in delays in the centralized information security review (CISR) process. The QRG instructs researchers on topics such as when CISR is required and what changes require ORR review.
In addition to the QRG, ORR developed an information sheet that describes the pilot ORR completed with the National Cancer Institute and VA Interagency Group to Accelerate Trials Enrollment (NAVIGATE) Program, which was showcased in last month's newsletter. VHA programs interested in establishing a similar arrangement with ORR can use the information sheet to get an idea of what is required.
Both documents are available to VAIRRS users through VAIRRS University.

The VAIRRS governance boards voted to develop an Administrative Checklist Wizard and Continuing Review/Closure Wizard. The Administrative Checklist will be the first board-facing wizard and will support administrative review. We will keep you updated on our progress over the summer. Email the VAIRRS Support Team at VAIRRS@va.gov for more information or if you would like to participate in the development process.

Please complete the VAIRRS Data Evaluation Survey by Wednesday, July 26, 2024. We request that individuals in the following roles at all VA research sites to please complete this survey:
The data collected will help shape the future of the VAIRRS program as we continue to enhance our services and support for all VA research sites.
A targeted email was sent to administrators at all sites on June 3, 2024. If you did not receive the email, please use the following link: https://forms.office.com/pages/responsepage.aspx?id=Ixtf6a-r7kWCHberJRqzv-oQQLNcNq9HtCyVCy1xValURDJZUk45Wkg3WEtOVjQ0UUUzTDJIREtOUC4u (VA network access only) Also, be sure to update the VAIRRS Distribution subscription list, so that you can receive important surveys and updates in the future.

VAIRRS has launched a new VAIRRS Distribution Subscription List (VA network access only), which will serve as the primary method to receive VAIRRS training announcements, surveys, and data-specific emails outside of the newsletters and program updates. With the launch of the subscription list, VAIRRS is retiring the Microsoft Outlook contact groups: VAIRRSAdministrators@va.gov and VAIRRSendusers@va.gov.
Please update your subscription using the VAIRRS Distribution Subscription List today. Select your VAMC and "subscribe to VAIRRS email."
Note: This subscription list does not include subscriptions to VAIRRS newsletters or other ORD news.

The new Data Curation Email Notification shares the data reported on the Data Curation page of the FSR Dashboard. This notification was sent to all VAMCs on Tuesday, June 18. Please forward these emails to any necessary points of contact for their information and rectification. To be included in these emails going forward, subscribe to the VAIRRS Distribution Subscription List.