Office of Research & Development |
 |
What is VA CURES?
VA CURES was initially established as a clinical trial master protocol framework to support and maximize the efficiency of COVID-19 clinical trials sponsored by Clinical Science Research and Development and the Cooperative Studies Program. The first trial, VA CURES1, of convalescent plasma, has been closed to recruitment.
What is the Goal of the VA CURES Program?
Since completing VA CURES1 (NCT04539275), the VA CURES Program has now turned attention to trials that can be completed with other partners, focusing first on partnership with NIH. The VA CURES Program will continue to focus on advancing clinical care of Veterans with SARS-CoV-2 infection by producing actionable data of the highest quality on strategies for novel treatments and prevention.
As an active clinical trials network, how does a site express interest in joining VA CURES Program for future studies?
Investigators may contact the program directly to express interest in current or future trials: CLIN-Review@va.gov
How is VA CURES organized?
The VA CURES Program is directed by an Executive Steering Committee. This committee comprises experts in various fields, including clinical trials design and operations, pulmonary & critical care medicine, and infectious diseases.
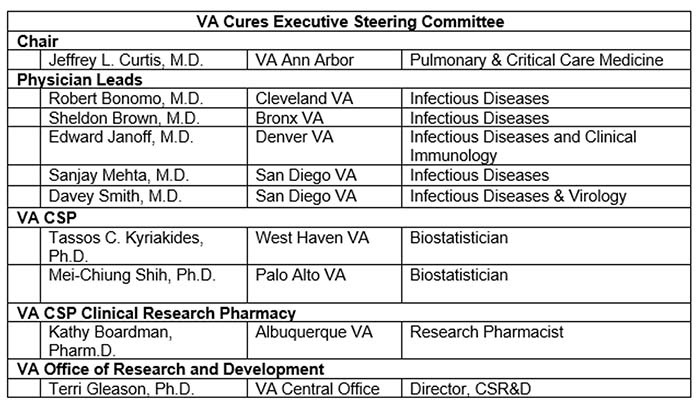
Who are the members of the VA CURES Executive Steering Committee?
What does the VA CURES Program do?
What studies are active or in planning under the VA CURES Framework?
|
Title |
Status |
|
In planning Completed |
ACTIVE-4a is an NHLBI-sponsored multicenter, randomized, controlled, adaptive platform trial investigating therapies for patients hospitalized for COVID-19. Its original focus was on antithrombotic strategies, but it has since expanded to other therapies designed to also reduce inflammation and prevent organ failure in SARS-CoV-2 infection.
Due to its adaptive protocol, the type and number of interventions in ACTIV-4a will change as the science evolves. With numerous global sites already participating, ACTIV-4a is anticipated to continue for years, regardless of local surges and lulls in the US.
If your VA site is interested in joining this collaboration, please contact Ashlea.Mayberry@va.gov expressing your interest. We anticipate our second wave of site enrollment to begin May 2022. Qualifying sites will be provided extensive training and rigorous support, including central VA Privacy and Information Security review for all VA sites, and aid with submitting the VA-approved protocol to ACTIV-4a’s commercial central IRB.