Office of Research & Development |
 |
The Department of Veterans Affairs (VA) Cooperative Studies Program (CSP) is a research organization in the Veterans Health Administration's Office of Research and Development. The CSP works with VA doctors to conduct clinical trials. The CSP also collaborates with others, such as the National Institutes of Health, universities and the pharmaceutical industry on these trials. The goal of clinical trials is try to compare and/or find treatments for diseases that affect our nation's veterans. Since many other people in the country also have these diseases, the results of CSP trials often improve and benefit the health of the United States population. In fact, CSP research has found better ways of treating diabetes, tuberculosis, high blood pressure, prostate cancer and many other illnesses.
The following information describes what clinical trials are and gives additional information to help you decide if participating in a clinical trial is right for you.
A clinical trial is an organized way of studying medical treatments for diseases. Medical treatments are medicines, devices or procedures. Clinical trials are research studies that help prove that medical treatments are safe and effective to use. They also can show whether one medical treatment is better than another.
At first, studies of new medicines are done in laboratories. If the medicine looks promising, it is then tested in animals. Usually, clinical trials are only started after the treatment is found to be safe in animals. Most clinical trials involve many people. This way, if the medical treatment is shown to be safe and effective, doctors will know what to expect in their patients.
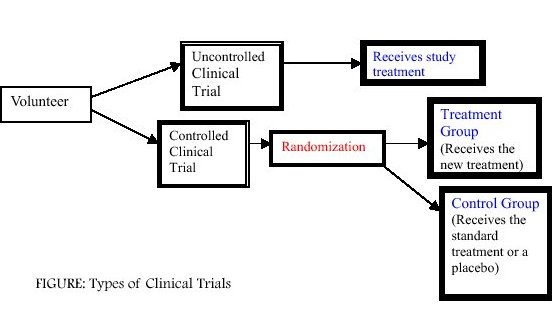
Generally, there are two types of clinical trials, uncontrolled and controlled. In an uncontrolled clinical trial, all participants simply receive the same treatment. However, these types of trials do not provide as much information as a controlled clinical trial.
In a controlled clinical trial, participants are divided into two groups. The first group, called the treatment group (or experimental group) may receive one or more kinds of treatment. The other type of group, called the control group, receives the standard treatment. Sometimes in medical trials, there is no standard treatment so a placebo or "sugar" pill is used. By dividing participants into groups, doctors can compare how well different treatments work.
An important part of a controlled clinical trial is that participants are randomly assigned to one of the two groups (see Figure). A participant who is randomly assigned means he or she is assigned to a group by chance (like a flip of a coin). A randomized clinical trial is the best way to study new treatments.
Often controlled clinical trials are also masked. There are two types of masked clinical trial designs, single masked and double masked. In a single masked clinical trial, participants do not know whether they received the new treatment or a standard treatment (or placebo) until the end of the study. In this type of clinical trial, the doctor and nurse know which treatment the participant received. In a double masked trial, neither the participant nor the doctor/nurse know whether the participant received the new treatment or a standard treatment (or placebo) until the end of the study. These clinical trial methods are important because they prevent people's behaviors or opinions from influencing the results of the clinical trial.
Clinical trials of medications involve four phases. Phase I clinical trials usually include between 20 to 80 healthy people. These clinical trials make sure a medication is safe. Phase II clinical trials are done only after a medication is shown to be safe. Phase II clinical trials involve no more than a few hundred participants who have the disease that the medication is intended to treat. These clinical trials are used to learn if the medication works and determines what dose should be used for the disease.
Phase III clinical trials can include up to several thousand participants. These clinical trials try to further confirm that the medication works for a specific disease. Phase III clinical trials also study the side effects of the medication. Both Phase II and Phase III clinical trials may compare the effectiveness of a new medication to a control group, who gets the standard treatment (or placebo). After a Phase III clinical trial is completed, data from some of the clinical trials can be sent to the Food and Drug Administration (FDA). If the FDA decides that the medication is safe and effective to use, it can then be marketed and prescribed by your doctor.
Once a medication is marketed, Phase IV clinical trials may be done. Phase IV clinical trials often include several thousand participants. These clinical trials provide additional information about the medication's effectiveness and safety. Specifically, these trials continue to collect information on the long-term side effects of a marketed medication. This information is later sent to the FDA.
Medical and surgical procedures, herbal remedies and dietary supplements are less regulated and do not go through this process.
Many important medical questions have been answered with clinical trials. These answers have helped improve medical care. Clinical trials usually lead to safer and more effective treatments for many diseases. They also show new directions for medical research that may lead to new therapies in the future.
VA clinical trials have found new therapies for heart failure, high blood pressure, coronary artery disease, stroke, tuberculosis, epilepsy, diabetes, kidney disease and gastrointestinal diseases.
There are many good reasons to take part in a clinical trial. In clinical trials, doctors discover new ways of curing or relieving symptoms of diseases. Many people choose to take part in a trial because the new treatment may help themselves or others.
In some clinical trials, a new treatment is found to be safer and more effective than other treatments available to your doctor. In this case, clinical trial participants are the first to benefit from it. However, there can be risks from new medical treatments, like side effects. Sometimes, however, a medication or treatment is less effective or even harmful. If the new treatment proves to be harmful or less effective before the trial is completed, your doctor will often decide to take you out of the clinical trial and do whatever is best for you. It is important that you know the benefits and risks of a new treatment before volunteering to participate in a clinical trial.
Informed consent is used for this purpose. This form describes the clinical trial and lists the benefits and risks of each treatment. Before any treatment is given, you must be told about these benefits and risks. You cannot be entered into a clinical trial until you sign the informed consent document.

Your rights as a volunteer in a clinical trial are protected in three ways. You must be adequately informed about the clinical trial. The clinical trial must be approved and continually monitored by an independent Institutional Review Board (IRB) or a Human Rights Committee (HRC) at your medical facility. An independent Monitoring Committee (DMC) established by the Cooperative Studies Program monitors each clinical trial.
The informed consent document that you must read and sign before you participate in a trial will explain the benefits and risks of the clinical trial.
Unique to the VA system is a Research and Development Committee at each VA medical center performing medical research, including clinical trials. This committee evaluates the scientific value of each research study performed at that VA. The committee's purpose is to make sure the research project will answer an important medical question. These committees provide another layer of protection for subjects participating in VA research projects.
Remember, you have the right to make your own decisions about your medical care. The decision to participate in a clinical trial is entirely yours. Talk with your doctor about all the medical treatments, including those being used in the clinical trial, that are available to you. This will help you make the best choices for your health.
| Institutional Review Board or Human Rights Committee | Data Monitoring Committee | |
| Members: |
|
|
| Purpose: |
|
|
| Reviews: |
|
|
| Evaluates studies for: |
|
|
| Will stop a trial if: (DMC only has authority to recommend stopping the clinical trial.) |
|
|
Informed consent is required for all clinical trials. It means that you have been told and understand exactly what will be done in the clinical trial. If you are interested in being participant, you will be given an informed consent form to read and think about carefully. This form must include the following items:
You have the freedom to decide whether you want to participate in a clinical trial. At this point, it is important to ask as many questions as you may have. After the doctor or nurse has answered all your questions completely, you can then decide whether or not to participate. You may take the informed consent form home and discuss it with family and friends before agreeing to participate. No one should pressure you to participate in any clinical trial. If you decide to participate, you must sign the informed consent form. If you decide not to participate, the doctor must treat you or refer you to your regular doctor for standard medical treatment. You do not have to explain why you choose not to participate. There are no penalties for not participating in a clinical trial. You do not have to participate in a clinical trial just because you signed an informed consent form. You may voluntarily withdraw from the clinical trial at any time.

Having an illness can be physically and psychologically stressful. Deciding whether to participate in a clinical trial is often an additional stress. The best way to feel comfortable about your decision to participate is to get as much information as you can. Be sure you understand the protocol and any tests you must take or samples you must give. Make sure you also understand the following:
You may have other questions too. Write them down so you can ask the doctor or nurse when you see them. You may involve others in your decision to participate in a clinical trial. The opinions of family members, friends and your regular doctor may be valuable to you. You may want to talk with others who are in the clinical trial. Ask if you can meet with other participants who are in the clinical trial. Remember, the final decision is yours.
Do not rush your decision. Make sure you feel comfortable about participating in the clinical trial. Remember, if for any reason you wish to leave a trial, you may do so at any time.
The sponsor of a clinical trial can be a person, a government agency (like the Department of Veterans Affairs or the National Institutes of Health), a university or a pharmaceutical company who wants to answer a specific research question. The sponsor is also responsible for the overall conduct of the clinical trial. The sponsor selects qualified doctors to perform the research and it may pay for the research. The sponsor also supplies detailed information to the doctors about the treatment being studied. Most importantly, the sponsor monitors the progress of the clinical trial and makes sure the protocol is followed.
Both healthy people and people with illnesses participate in clinical trials. Healthy people are involved in Phase I studies, which determine the safety of a medication. For example, clinical trials of vaccines (like the polio vaccine) often include healthy people. These people are usually at risk for getting the disease the vaccine is meant to prevent. Most clinical trials, however, involve people with a specific illness who may be helped by the new treatment. These kinds of clinical trials are usually Phase II, Phase III and Phase IV trials.
There are often restrictions that specify who can be included in a clinical trial. The clinical trial protocol will specify which participants can be included. The clinical trial doctor will determine if you are eligible to participate in the trial.
While participating in a clinical trial, you may receive more medical care than you would normally receive. Many clinical trials may require that you have more tests and/or doctor visits than usual. People participating in clinical trials are often seen by the clinical trial doctors and nurses in the same places they receive their regular medical care. You should not have to pay for any treatments or tests that you receive for participating in a clinical trial.
You may also have to keep records or fill out forms about your health. The tests, visits and time involved are described in the informed consent form. The visits may include an exam of your health at the beginning of the clinical trial and several visits during the trial, often referred to as patient follow-up. The clinical trial doctor or nurse may also keep in touch with you for a while after the treatment in the trial is over.
Patient adherence is how well a patient follows the study doctor and nurse's instructions. If you do not follow these instructions, the results of the clinical trial may not be valid. For example, if you do not take the clinical trial medication being studied as directed, the medication may appear not to work when it really does work. The result may be to deny you and other participants the treatment that is needed. Therefore, patient adherence to the clinical trial instructions and procedures is extremely important to all of us. If you are unable to perform any of the protocol procedures or take the clinical trial medication, you should inform the clinical trial doctor or nurse, especially if you experience any side effects from the treatment.

Clinical trials are very important in improving health and the care provided to patients. We have given you some basic information about clinical trials. You may have other questions about clinical trials that the CSP is planning or conducting. If you do, please ask your doctor or nurse or visit studies section of this site.
Adherence - How well participants follow the treatment plan in a clinical trial.
Clinical trial - A study of medical treatments (medicines, devices or procedures).
Clinical trial medication - The medication being tested in a clinical trial.
Control group - The group of clinical trial participants who do not receive the new treatment. These participants receive either a placebo or standard therapy.
Controlled clinical trial - A clinical trial that involves dividing participants into two groups, treatment and control. In this type of clinical trial, participants are randomly assigned to one of the two treatments.
Data Monitoring Committee (DMC) - A committee that reviews data from a clinical trial to see if there is clear harm or benefit for participants while a clinical trial is still on-going. This committee has the ability to stop a clinical trial early if necessary.
Double masked clinical trial - A clinical trial in which neither the participant nor the clinical trial doctor/nurse know whether the participant is receiving the new treatment or a standard treatment (or placebo).
Experimental group - The group of clinical trial participants that receive the new treatment.
Food and Drug Administration (FDA) - The government agency responsible for making sure that drugs and medical devices are safe and effective.
Informed consent - A participant's right to know all the clinical trial's instructions and procedures, all the risks and benefits of being in the clinical trial and have all his/her questions answered before choosing whether to participate in the clinical trial. This information must be given to the participant both verbally and in writing. Before a participant can participate in a clinical trial, the participant must sign the clinical trial's informed consent form. By signing the informed consent form, the participant acknowledges that he/she understands what participation in the clinical trial will involve and has made a decision to participate. See the section "What is Informed Consent?" for a further explanation.
Institutional Review Board (IRB) or Human Rights Committee (HRC) - A committee that is not a part of the clinical trial. This committee reviews and approves protocols to make sure that participants are not exposed to unnecessary or excessive risks.
Medical device - A product that is made or constructed for a particular medical need or purpose (hearing aid, dental implants, intraocular lens, etc.).
Phase I clinical trial - A clinical trial that tries to show the initial safety of a new medication. These clinical trials are usually done in healthy volunteers.
Phase II clinical trial - A clinical trial that tries to see if the medication can work in participants with the disease to be treated.
Phase III clinical trial - Phase IV clinical trial/Post-marketing surveillance participants with the disease to be treated.
Phase IV clinical trial/Post-marketing surveillance - A clinical trial that is carried out after a medicationis marketed or approved for licensing by the FDA. It evaluates the medication's long-term effects.
Placebo - A tablet or capsule that contains no medication.
Protocol - A document that tells the clinical trial doctors how the clinical trial will be conducted.
Randomization - The process by which a clinical trial patient is assigned to a treatment group by chance (like by the flip of a coin). Participants are randomly assigned to receive either the new treatment or a standard treatment (or placebo).
Research and Development Committee - A committee formed at each VA facility doing medical research. This committee is responsible for the review of the design of all research projects involving humans. This committee also works closely with the Institutional Review Board at the VA facility to make sure all legal and ethical issues are reviewed.
Side effect - Any effect caused by a medication other than what was intended when a patient takes a medicine.
Single masked clinical trial design - A clinical trial in which the participant does not know whether he/she received the new treatment or a standard treatment (or placebo) until the end of the clinical trial. In this type of clinical trial, the doctor and nurse are aware of which treatment the patient is receiving.
Sponsor - The person, agency, university or pharmaceutical company who pays for the clinical trial.
Standard therapy/Standard treatment - The usual treatment for an illness to which a new treatment may be compared. This is also known as the control.
Treatment group - The group of clinical trial participants that receive the new treatment.
Treatment plan - The way the medication will be used in a clinical trial. The plan is described in the clinical trial's protocol.
Uncontrolled clinical trial - A clinical trial in which clinical trial doctor and nurse and the participant know what medications are being used. This is also called an open-label clinical trial .