Office of Research & Development |
 |
Office of Research & Development |
 |
 The Prostate Cancer, Genetic Risk, and Equitable Screening Study (ProGRESS), conducted by the Department of Veterans Affairs (VA), aims to explore if genetic testing can improve prostate cancer screening. The study uses genetic information from saliva to measure a participant’s risk of developing prostate cancer. Enrolled participants are randomized to receive either standard of care or precision prostate cancer screening information recommendations tailored to their individual genetic profile. This information is provided to participants and their healthcare providers. This precision approach is not yet a part of routine prostate cancer screening but might help target screening to individuals at highest risk of developing prostate cancer while safely deferring screening for low-risk individuals.
The Prostate Cancer, Genetic Risk, and Equitable Screening Study (ProGRESS), conducted by the Department of Veterans Affairs (VA), aims to explore if genetic testing can improve prostate cancer screening. The study uses genetic information from saliva to measure a participant’s risk of developing prostate cancer. Enrolled participants are randomized to receive either standard of care or precision prostate cancer screening information recommendations tailored to their individual genetic profile. This information is provided to participants and their healthcare providers. This precision approach is not yet a part of routine prostate cancer screening but might help target screening to individuals at highest risk of developing prostate cancer while safely deferring screening for low-risk individuals.
Prostate cancer screening, most commonly by a prostate-specific antigen (PSA) test, modestly reduces prostate cancer related deaths but also increases the risks associated with overdiagnosis and overtreatment. As a result, there is no universal prostate cancer screening recommendation from the United States Preventive Services Task Force (USPSTF) or the VHA National Center for Health Promotion and Disease Prevention.
Genetic testing is advancing precision prostate cancer screening. An individual's genetic profile may estimate a person’s risk for developing prostate cancer, including risk of metastatic or even lethal disease. By joining ProGRESS, participants will play a pivotal role in the quest to help researchers better understand and potentially improve prostate cancer screening for Veterans for generations to come.
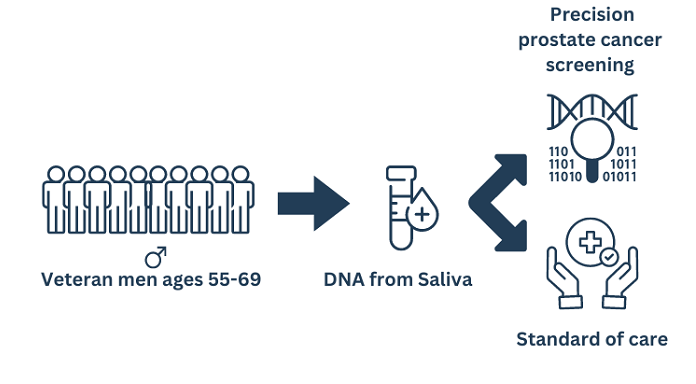
The study will identify eligible participants, Veterans between the ages of 55-69 who have not previously had a prostate biopsy or MRI and who have not been diagnosed with prostate cancer. Recruitment outreach will include home mailings, emails, phone calls, and public announcements. We expect to enroll a diverse sample of approximately 5,000 Veterans nationwide.
There are two ways a participant can consent to participate in the study:
Participants will provide a one–time saliva sample via a saliva collection kit that is delivered directly to their home. The self-addressed kit will be sent from and returned to Broad Clinical Labs for DNA extraction, genetic analysis, and report generation.
The Million Veteran Program (MVP) is a national research initiative by the Department of Veterans Affairs that aims to understand how genes, lifestyles, military experiences, and exposures affect health and wellness. The ProGRESS Study team has used data from MVP to develop the Prostate CAncer integrated Risk Evaluation (P-CARE) model comprised of 601 genetic variants, genetic ancestry, and patient-reported family history of prostate cancer. P-CARE results may be associated with a high, average, or low risk of developing metastatic prostate cancer.
Enrolled participants who provide a viable biospecimen (saliva) will be randomized into one of two groups: the precision screening intervention arm or standard of care arm of the trial.
All subjects will participate in prostate health surveys conducted at regularly for up to 10 years.
Broad Institute - Performing genetic analysis.
VA Salt Lake City, VA San Diego / University of California, San Diego - Developed the PCARE Model
Resources:
If you have any questions, comments, or concerns about the research please contact ProGRESS at 833-607-5281 or by email ProGRESS@Va.gov. Additionally, if you have any questions about your rights as a study participant, or you would like to confirm this is a valid VA study, you may contact the VA Central Institutional Review Board (IRB) toll free at 877-254-3130.