Office of Research & Development |
 |

VA Research Currents archive
March 5, 2014

Veteran Glenn Cuff has served as a diabetes peer mentor to other Veterans. (Photo by Tommy Leonardi)
A landmark trial to determine the long-term effectiveness of drugs used to treat type 2 diabetes will involve nine VA centers, along with 37 other clinical sites.
The Glycemia Reduction Approaches in Diabetes (GRADE) trial, funded by the National Institutes of Health, aims to learn which of four widely used, well-established diabetes drugs is most effective for which patients. The study is expected to include some 5,000 participants nationwide.
"It's the first time these drugs have been compared head to head," says Dr. Sophia Hazel Hox, an osteopathic endocrinologist who will help lead the trial at the VA Pacific Health Care System in Hawaii. "The hope is that we'll be able to gather information on which drug or drug combination is best for a given population—not just on treatment, but as far as avoiding adverse side effects as well."
The other VA sites are Atlanta, Cincinnati, Cleveland, Denver, Miami, Omaha, San Diego, and Seattle. Each clinical center in the study has a target enrollment of 150 participants.
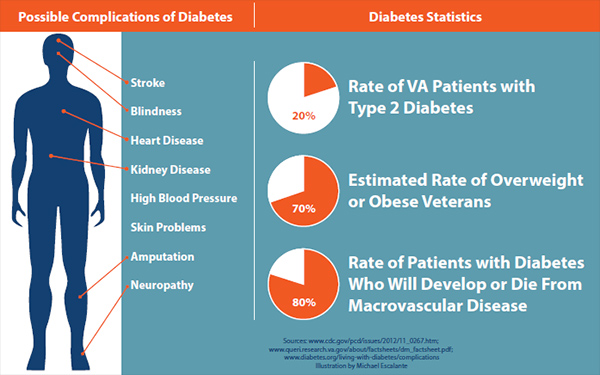
Type 2 diabetes affects nearly 30 million Americans nationwide. About one in five VA patients has the condition.
The GRADE study is open to patients over age 30 who have been diagnosed with type 2 diabetes in the past five years.
The four medications to be compared are sitagliptin (sold as Januvia), glimepiride (Amaryl), liraglutide (Victoza), and glargine (Lantus). Participants will take one of the four drugs in concert with metformin (Glucophage), a drug typically used as a firstline treatment.
Researchers will examine the participants and collect data quarterly over the next four to seven years. Participants will be advanced to insulin therapy if the initial combination is not adequate.
The randomized, controlled study is expected to yield evidence to help providers make educated, fact-based decisions on diabetes care.
Several physician societies have developed guidelines for the management of diabetes based on the available data. Doctors have typically used these guidelines to determine what medications to prescribe to patients with type 2 diabetes. Researchers are hoping GRADE will provide data for more robust algorithms.
Hazel Hox: "Some of these drugs have been on the market for 10 years or more, and while we know they work, we don't know if one is better than another or if there is a particular scenario where one is more optimal. There are many options for doctors today as far as what treatment option they choose. It often comes down to the circumstances and health issues at the time the patient is in the office and what medications they are familiar with."
She adds that GRADE should move VA and other providers one step closer to personalized medicine for patients with diabetes.
"Knowing that we have a large and growing population of people suffering from diabetes, we need to be doing our best to treat them with the most effective care. Part of that is giving them the right medication," she says. "This is one piece of that puzzle. The results of this study will get us closer to the kind of tailored, personalized medicine we need to provide."
For more information about the study, contact the study team at (216) 231-3240 or visit the website at https://grade.bsc.gwu.edu.