Office of Research & Development |
 |
Office of Research & Development |
 |

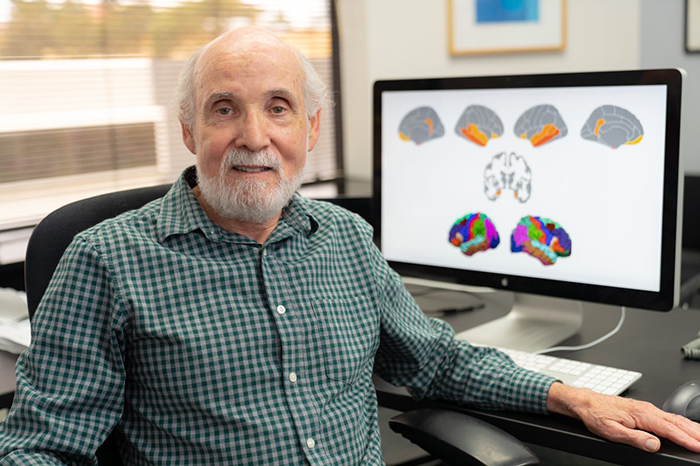
Dr. William Kremen, a neuropsychologist, embarked on the study because little is known about the ability of brain images of cognitively normal adults to predict progression to mild cognitive impairment. (Photo by Kyle Dykes, UC San Diego Health)
October 12, 2021
By Mike Richman
VA Research Communications
"The pathological process in Alzheimer's disease begins 20 or more years before dementia onset. Identifying risk factors in cognitively unimpaired middle-aged adults may be a step toward earlier intervention."
A new study shows that brain images of cognitively normal adults in their mid-50s can help in predicting the progression of mild cognitive impairment more than a decade later.
Mild cognitive impairment is a sign of early memory loss or other cognitive disabilities and can be a precursor to Alzheimer’s disease, one of the most common forms of dementia.
The results appeared in the journal Brain Communications in July 2021.
Could cholesterol medicine reduce dementia risk in seniors?

VA study reveals dementia risks unique to people with African ancestry
Head trauma, PTSD may increase genetic variant's impact on Alzheimer's risk
VA-led research finds PET scans important for ruling out Alzheimer's disease
Dr. William Kremen, a neuropsychologist who is affiliated with the Center of Excellence for Stress and Mental Health at VA San Diego, led the study. He embarked on the research because little is known about the ability of brain images of cognitively normal adults to predict progression to mild cognitive impairment. Most of the research on brain images in the study of Alzheimer’s disease—referred to as Alzheimer’s disease signatures in the paper—has involved adults over 70 years of age.
“The pathological process in Alzheimer’s disease begins 20 or more years before dementia onset,” says Kremen, who is also a professor of psychiatry at the University of California, San Diego. “Identifying risk factors in cognitively unimpaired middle-aged adults may be a step toward earlier intervention.”
The study included 169 cognitively normal male Veterans with an average age of 56. In the main finding, Kremen and his team learned that a loss of structural integrity, as indicated by the images, significantly improved the prediction of progression to mild cognitive impairment over a 12-year follow-up period. A loss of structural integrity in brain tissue can be a sign of neurodegeneration.
The research team, which used magnetic resonance imaging (MRI) to produce the brain images, based its finding “on a continuous measure to observe the degree of structural integrity from better to worse,” Kremen notes. “We’re not at the stage yet where we can have a set cutoff for abnormality. It’s an index of what’s called microstructural integrity. Our results suggest that poorer structural integrity tends to be associated with a higher risk for developing mild cognitive impairment later on.”
In a secondary analysis, Kremen and his colleagues looked at brain images while controlling for the effects of general aging. They observed that the Alzheimer’s-related brain regions remained as significant predictors, suggesting that the effect was not simply due to general aging.
The researchers used grey matter images to predict the progression to mild cognitive impairment. Grey matter is a key component of the central nervous system that helps people control movement, memory, and emotions. Grey matter images, the research team believed, would be better predictors of mild cognitive impairment than those based on cortical thickness or the volume of the hippocampus.
Cortical thickness is a measure used to describe the combined thickness of layers of the cerebral cortex, which is involved in complex brain functions, such as language and information processing. The hippocampus is a brain region that is important for its involvement in memory. One of the most consistent features of Alzheimer’s disease is atrophy in certain brain regions, particularly the hippocampus. Prior research, Kremen says, has used images based on measurements of grey matter thickness and volume.
In Kremen’s study, the researchers used an Alzheimer’s brain signature that they previously developed with data from the Alzheimer’s Disease Neuroimaging Initiative, a multisite study that aims to improve clinical trials for preventing and treating Alzheimer’s. The signature, a weighted average of thickness in seven cortical regions plus the volume of the hippocampus, was published about a decade ago in a study led by Dr. Linda McEvoy, a scientist at the University of California, San Diego. She was a co-author of Kremen’s 2021 paper.
Kremen’s team then created a novel brain signature based on grey matter diffusivity. Mean diffusivity is the average mobility of water molecules in the brain tissue.
To create the mean diffusivity signature, the researchers used the same regions and weightings as those in McEvoy’s paper. But they applied them with a measure of grey matter mean diffusivity in each of the regions. This type of signature was a better aid to predicting mild cognitive impairment than the signature based on thickness and volume, Kremen notes. “Grey matter mean diffusivity signatures may serve as useful imaging biomarkers that are sensitive to Alzheimer’s disease-related changes earlier than thickness or volume measures,” the researchers write.
Kremen: “The more directional the movement of water is, the more integrity to the structure. When the direction of the water molecules is more diffuse, then we assume there’s less integrity to the structure. If there’s more integrity in the structure, it’s confining the water molecules to move in a particular direction. The more diffuse those get, then that’s the idea.”
Each of the 169 participants in the study was part of the Vietnam Era Twin Study of Aging (VETSA), an NIH-funded project conducted in conjunction with VA. The project is a study of cognitive aging of male twins who served in the U.S. military during the Vietnam conflict from 1965 to 1975. Kremen has led VETSA for nearly two decades.
“The young age of the sample at baseline is particularly notable,” Kremen and his research team write. “Given that the brain signatures were examined when participants were only in their 50s, our results suggest a promising step toward improving very early identification of Alzheimer’s disease risk and the potential value of mean diffusivity and-or [multiple] brain signatures.”
Kremen, whose research focuses on cognition, aging, and genetics, says mild cognitive impairment is not always a precursor to Alzheimer’s disease or other dementias. “Some cases of mild cognitive impairment revert back to normal cognitive function, and some may remain as mild cognitive impairment without progressing to dementia,” he explains, noting that factors such as stress, depression, and effects from other diseases or drugs can cause temporary cases of mild cognitive impairment.
Alzheimer's disease, which accounts for 60% to 80% of all diagnosed dementia cases, is a progressive disease that involves the deterioration of nerve cells in the brain, which in turn affects thoughts, memory, and language.
Under Kremen’s supervision, clinical psychology doctoral student McKenna Williams, first author of the study, plans to do a follow-up. It will create a new mean diffusivity signature that may not necessarily include the same brain regions as the signature based on thickness and volume.
“But because diffusivity is a measure of something different about the grey matter,” Kremen says, “we don’t know if a new diffusivity signature created from scratch to differentiate Alzheimer’s cases and controls would involve exactly the same brain regions.”
Meanwhile, Kremen and his colleagues are following the VETSA sample to learn about the outcomes related to mild cognitive impairment and dementia at different stages of life and what midlife factors predict those outcomes. His 2021 study covered three waves: a baseline at the average age of 56 to predict progression to mild cognitive impairment and two ensuing six-year waves. His team used the latest available wave (wave three) as the outcome for mild cognitive impairment versus cognitively unimpaired status.
The researchers are now in the early stages of wave four. The average age of the participants is about 74.
“We’re highly likely to see more mild cognitive impairment and dementia, but at that average age there’s still going to be only a few cases of dementia,” he says. “But that’s part of our research. We started seeing people early in midlife, because we want to get a handle on what predicts the risk for dementia before people reach the full-blown stages of dementia. The earlier we can identify the risk, the more chance we have of being able to intervene in some way to possibly slow the progression. Once people have dementia, there’s too much pathology to do much that’s effective.”
VA Research Currents archives || Sign up for VA Research updates