Office of Research & Development |
 |

VA Research Currents archive
July 21, 2016
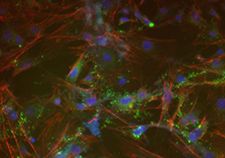
This microscope image shows trabecular meshwork cells created from induced pluripotent stem cells in the lab of Dr. Markus Kuehn. (Image courtesy of Kuehn lab)
An infusion of stem cells could help restore proper drainage for fluid-clogged eyes at risk for glaucoma. That's the upshot of a study led by a VA and University of Iowa team.
Researchers led by Dr. Markus Kuehn injected stem cells into the eyes of mice with glaucoma. The influx of cells regenerated the tiny, delicate patch of tissue known as the trabecular meshwork, which serves as a drain for the eyes to avoid fluid buildup. When fluid accumulates in the eye, the increase in pressure could lead to glaucoma. The disease damages the optic nerve and can result in blindness.
"We believe that replacement of damaged or lost trabecular meshwork cells with healthy cells can lead to functional restoration following transplantation into glaucoma eyes," writes Kuehn on his lab's website.
His group reported their findings in the June 21, 2016, issue of the Proceedings of the National Academy of Sciences.
Eye doctors use a variety of tests to catch signs of glaucoma. Early detection and prompt treatment can prevent vision loss, but the condition is chronic and must be monitored for life. (Photo by Steven E. Smith)
One potential advantage of the approach is that the type of stem cells used—called induced pluripotent stem cells—could be created from cells harvested from a patient's own skin. That gets around the ethical quandary of using fetal stem cells, and it also lessens the chance of the patient's body rejecting the transplanted cells.
Kuehn's team was able to get the stem cells to grow into cells like those of the trabecular meshwork by culturing them in a solution that had previously been "conditioned" by actual human trabecular meshwork cells.
The researchers were encouraged to see that the stem cell injection led to a proliferation of new endogenous cells within the trabecular meshwork. In other words, it appears the stem cells not only survived on their own, but coaxed the body into making more of its own cells within the eye, thus multiplying the therapeutic effect.
The team measured the effects in the mice nine weeks after the transplant. Lab mice generally live only two or three years, and nine weeks is roughly equal to about five or six years for humans.
Some 120,000 Americans are blind from glaucoma, according to the Gluacoma Research Foundation. African Americans are at especially high risk, as are people over age 60, those with diabetes, and those with a family history of the disease. The disease is not curable, but it can be managed so as to prevent the eventual loss of vision. Among the treatments currently used are eye drops and laser or traditional surgery.
The researchers say they are confident that their findings hold promise for the most common form of glaucoma, known as primary open angle glaucoma. They aren't sure yet if their mouse model is as relevant for other forms of the disease.
Another possible limitation of the research: It could be that new trabecular meshwork cells generated from the stem cell infusion eventually succumb to the same disease process that caused the breakdown in the first place. This would require retreatment. It's unclear, though, whether an approach requiring multiple treatments over time would be viable. The researchers plan to continue studying the approach.
Kuehn leads the Glaucoma Cell Biology Laboratory at the University of Iowa Carver College of Medicine, and is an investigator at the Center for the Prevention and Treatment of Visual Loss at the Iowa City VA Health Care System.